Choose from 228 different sets of melting freezing flashcards on quizlet. And while you can melt and freeze water indefinitely without any noticeable changes the same is not true of margarine and chocolate.
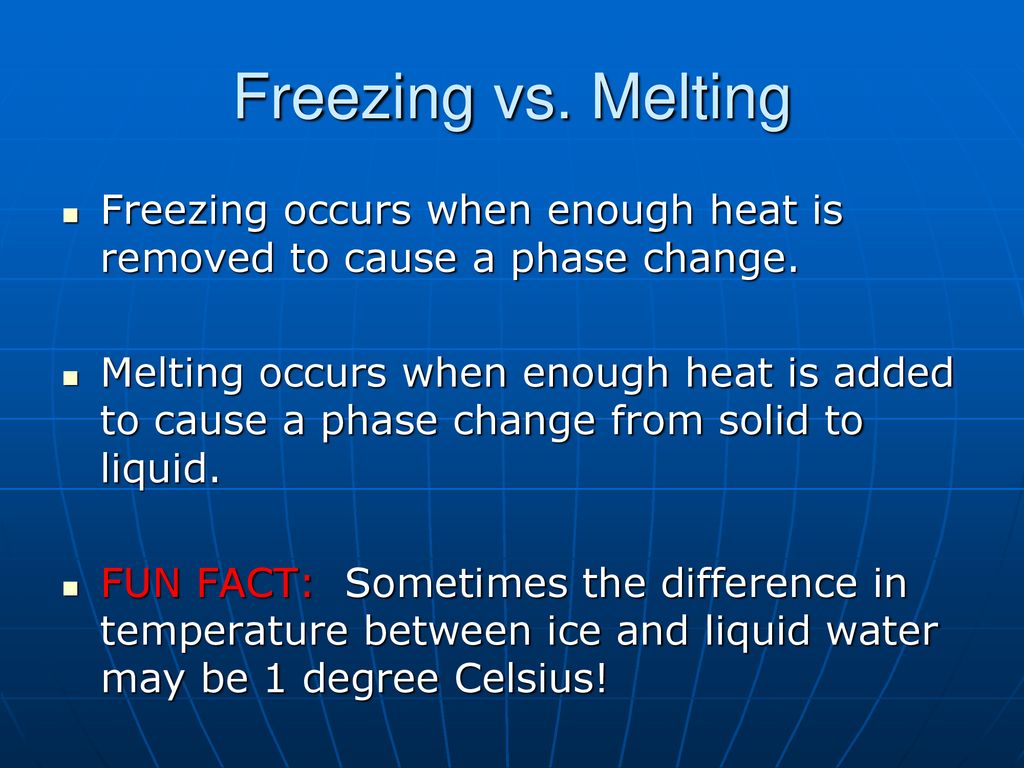 Boiling Freezing And Melting Water Ppt Download
Boiling Freezing And Melting Water Ppt Download
4114 heating and changes of state.

Melting And Freezing Mobi Download. 411 states of matter. Freezing and melting freezing is the change that occurs when a liquid changes into a solid as the temperature decreases. A pure substance has the same freezing and melting points in practice a small difference between these quantities can be observed.
Removing heat causes water a liquid to freeze to form ice a solid. The key difference between melting point and freezing point is that melting point is the temperature at which a solid will go to the liquid state whereas freezing point is the point where any liquid will change its state to a solid. Learn melting freezing with free interactive flashcards.
Gradual change of state from solid to liquid by absorbing heat from surroundings is called melting. Describe how heating a system will change the energy stored within the system and raise its temperature or produce changes of state. The melting and freezing point changes with pressure but normally they are given at 1 atm.
These are both examples of changes in the states of matter of substances. Freezing and melting solids and liquids can be changed from one state to another by heating or cooling. When water changes to a solid or a gas we say it changes to a different state of matter.
For example water has a specific freezing and melting point whereas margarine and chocolate do not. Adding heat can cause ice a solid to melt to form water a liquid. Melting point and freezing point are two physical properties of substances.
Heat melts a solid and turns it into a liquid. Melting and freezing take place at the melting point boiling and condensing take place at the boiling point. Water can exist as a solid ice liquid water or gas vapour or gas.
The temperature at which solid transforms to liquid is called melting point of the concerned substance. Gradual change of state from liquid to solid by leaving heat to surroundings is termed as freezing. Melting is the opposite change from a solid to a liquid as the temperature increases.
Cooling freezes a liquid into a solid.
 What Are Freezing And Melting Bbc Bitesize
What Are Freezing And Melting Bbc Bitesize
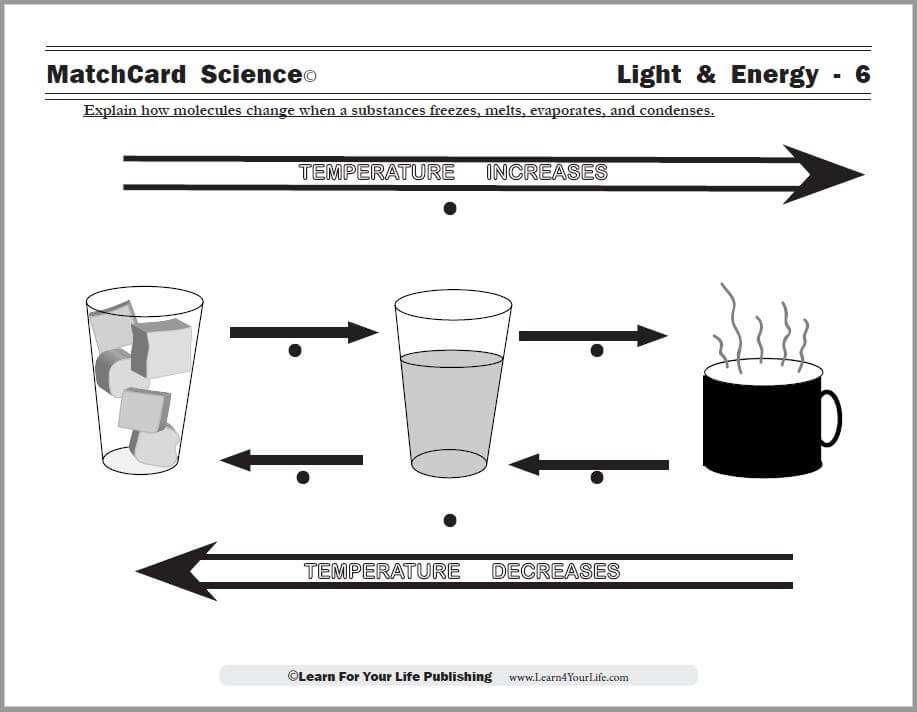 Evaporation And Condensation
Evaporation And Condensation
 Changes Of Phase List The Four Phases Of Matter In Order Of
Changes Of Phase List The Four Phases Of Matter In Order Of
 Multimedia Changing Statemelting Chapter 2 Lesson 5
Multimedia Changing Statemelting Chapter 2 Lesson 5
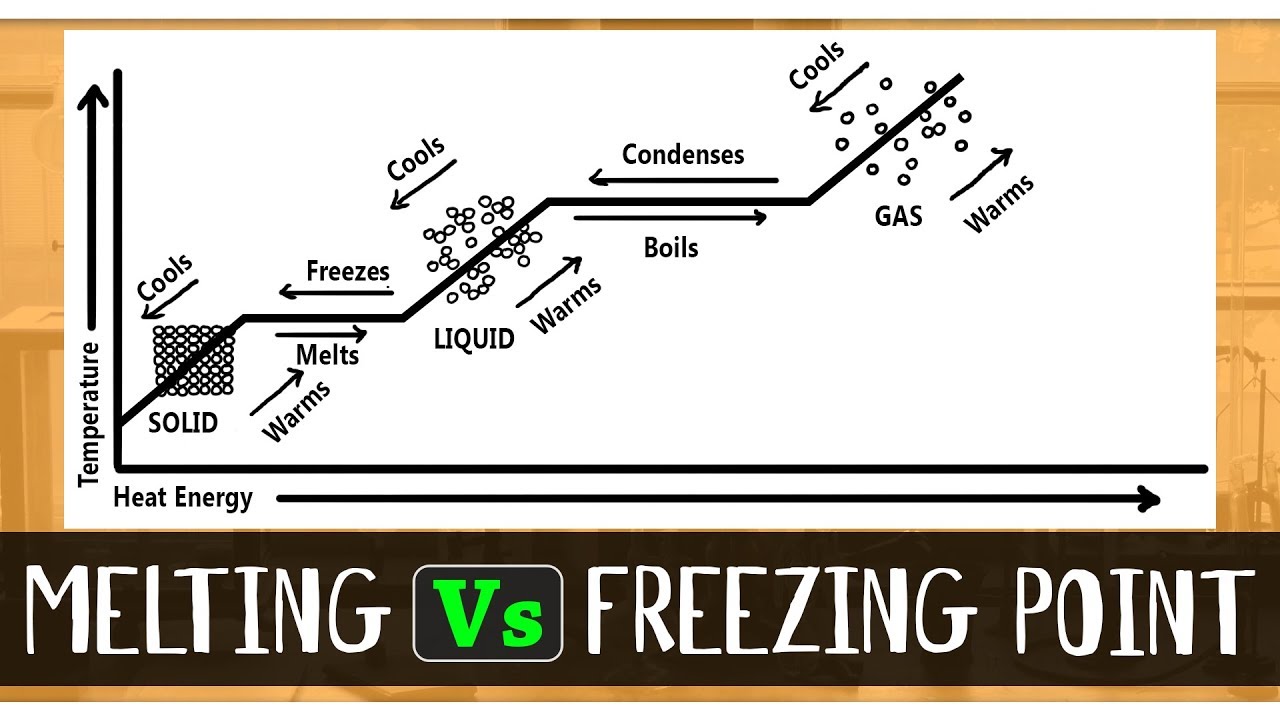 What Is The Difference Between Melting And Freezing Point Chemistry Concepts
What Is The Difference Between Melting And Freezing Point Chemistry Concepts
Gcse Chemistry Changing From A Solid To A Liquid To A Gas
Freezing Melting Points Lessons Tes Teach
 What Is Meant By The Kinetic Theory Of Matter A Plus Topper
What Is Meant By The Kinetic Theory Of Matter A Plus Topper

 Dsc Plot Of Melting And Freezing Profiles Of Experimental
Dsc Plot Of Melting And Freezing Profiles Of Experimental
 Melting And Freezing Quiz Proprofs Quiz
Melting And Freezing Quiz Proprofs Quiz
 Worksheet Worksheet Melting Points Melting Points
Worksheet Worksheet Melting Points Melting Points
 Freezing And Melting Lerner Publishing Group
Freezing And Melting Lerner Publishing Group
Melting Point Lessons Tes Teach
 Thermodynamics Of Freezing And Melting Nature Communications
Thermodynamics Of Freezing And Melting Nature Communications
0 Response to "Melting And Freezing Ebook Download"
Posting Komentar